Green Energy
Low-cost CO2 to enable the transition from fossil fuels to e-fuels
Dec 19 2023
Author credit – Stephen B. Harrison, sbh4 consulting
At COP28 in the United Arab Emirates, the need to transition away from fossil fuels was reiterated in the closing agreements. The Middle East is a region blessed with the potential to generate many GW of low-cost renewable electricity from wind and solar. This can be used to create green hydrogen using electrolysis of water.
Reacting green hydrogen with low-cost captured CO2 can result in synthetic aviation fuel or e-methanol for shipping. To embark upon such a path, the question will become how to access an abundant source of affordable CO2 within the region.
CO2 capture is essential in ethylene oxide (EO) and ethylene glycol (EG) production to avoid an accumulation of CO2 in the reactor gas recycle loop. This CO2 can be utilised to produce synthetic aviation fuel through the power to liquids pathway and other e-fuels. CO2 captured from EO production must surely be low-hanging fruit when it comes to scalable, accessible, low-cost source of CO2 for e-fuels.
CO2 is captured from one of Equate Petrochemical Company’s EO/EG plants in Kuwait’s Shuaiba Industrial Area. The facility captures 55,000 tonnes of CO2 per year. A similar CO2 capture facility operates at Petro Rabigh in Saudi Arabia. The plant was brought into operation in 2023 and can capture 100,000 tonnes per year of CO2. These, and other CO2 sources from hydrogen or ammonia production can readily be used for e-fuels production in the Middle East.
EO as a petrochemical building block
Around 20 million tonnes of EO are produced each year. The EO molecule reacts easily to form other hydrocarbons. The majority of this EO is converted to monoethylene glycol (MEG) and polyethylene glycol (PEG).
PEG is an antifreeze used at airports to defrost aircraft and in car windscreen washer fluids. MEG is the precursor for the plastic PET, which is commonly used to make drinks bottles and polyester fibres for textiles. EO can also be converted to ethanolamines, which are used as solvents for post-combustion CO2 capture.
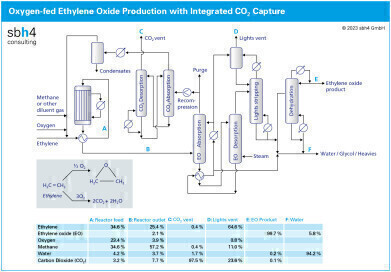
The modern process to produce ethylene oxide (EO) combines pure oxygen with ethylene. The reaction takes place at around 20 bar pressure and 250 °C. The ethylene concentration in the reactor ranges from 35% by volume at the inlet to 25% at the exit.
Commercial EO production relies on silver catalysts
The catalyst used for EO production is based on silver oxide catalyst mounted on an alumina support. EO catalysts are characterised by several performance factors, including conversion, selectivity, activity, productivity, and stability. Selectivity represents the amount of ethylene converted to EO compared to the total amount of ethylene reacted to EO, CO2 and other hydrocarbons.
Conversion refers to the amount of ethylene that reacts during each pass over the catalyst. Since the conversion is not complete, gases leaving the reactor are recycled back through the reactor to ensure the valuable feedstock is fully utilised.
Whist the role of the catalyst is to selectively produce EO, some CO2 formation during the reaction is inevitable. Since the some of the reactor outlet gases are recycled to the reactor inlet maximise the conversion of ethylene, CO2 must be removed from the process to avoid accumulation. This is achieved with a CO2 capture loop using hot potassium carbonate (HPC) as the solvent.
CO2 capture technology selection
Gases leaving the EO reactor are generally at around 20 bar and contain around 4% of oxygen and 8% CO2. HPC is an ideal CO2 capture solvent for selectively removing CO2 from high pressure gas mixtures such as this. Unlike amine-based CO2 capture solvents, HPC is not degraded by the oxygen present in this stream.
The equipment to capture CO2 is generally a twin tower system. In the first tower, which operates at high pressure, CO2 is absorbed into the HPC solvent. The high pressure helps to drive the gas into the liquid. Prior to entering the second tower, the pressure of liquid containing the gas is reduced, or flashed. This releases much of the CO2 without the need to apply heat energy.
The solvent loaded with CO2 is boiled at the base of the second tower to drive out the CO2. This is where the energy to the CO2 capture process must be applied. The regenerated solvent from the base of the second tower is pumped up to the pressure where it can enter the first tower to absorb more CO2.
The gas leaving the CO2 stripper column is rich in CO2, with water vapour being the main additional component. Much of the water vapour can be removed simply by cooling followed by and gas / liquid phase separation in knock-out drum. This stream is ideal to feed directly to a CO2 liquefier.
Hydrogen and ammonia production as low-cost CO2 sources
Beyond its application in EO production, HPC is commonly used to capture CO2 from steam methane reformer (SMR) syngas, which is at a similar pressure to the EO process. CO2 must be removed in this application prior to the hydrogen being used for ammonia production. There is no oxygen in the SMR syngas stream, so it is possible to add some organic promoters such as ethanolamine to the HPC solvent to increase the CO2 capture rate in this application.
CO2 captured from SMRs used to generate hydrogen on refineries and in the ammonia, fertilizers can also be a cost-effective source of CO2 for e-fuels. During ammonia production, like the EO process, CO2 must be removed to protect the ammonia synthesis catalyst.
Events
IWA World Water Congress & Exhibition
Aug 11 2024 Toronto, Canada
Aug 25 2024 Stockholm, Sweden and online
Sep 03 2024 Mexico City, Mexico
Sep 03 2024 Mexico City, Mexico
Sep 03 2024 San Diego, CA, USA