Hazardous Waste
Recover Metals from Weak Acid
Jun 14 2012
Eisenmann (Germany) has developed a new precipitation process for recovering copper and other materials from the purification of exhaust gases from copper smelting plants.
Exhaust gases from copper smelting contain heavy metals such as copper, arsenic, iron, mercury, lead, zinc, molybdenum, cadmium, sulphur dioxide, and sulphur trioxide. These substances are washed out when the gas is treated. The wet process produces several cubic meters per hour of liquid weak acid containing up to 35 percent sulphuric acid. “This often contains such a high concentration of copper and sulphuric acid, two valuable raw materials, that it makes financial sense to recover these substances from the liquid,” explains Angela Ante, waste water treatment expert at plant engineering specialist Eisenmann, headquartered in Böblingen, Germany. “Standard procedure until now has been to neutralise the weak acid along with all the reusable contents using milk of lime, and then dispose of the resulting sludge at a high cost.”
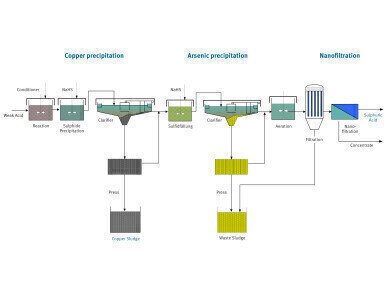
The new precipitation method developed by Eisenmann involves stirring sodium hydrosulphide (NaHS) into the weak acid, inducing precipitation of the heavy metals. “However, if no other special measures are taken when the NaHS is added, the copper we want and the hazardous pollutant arsenic are precipitated almost simultaneously,” describes Ante. “To avoid this, we add another chemical catalyst at the beginning of the process.” By doing so, the copper and arsenic are precipitated in distinct, successive phases. The copper sludge extracted in the first precipitation reactor is mechanically dewatered and can subsequently be fed directly back into the smelting process. Every liter of weak acid can contain as much as 18 grams of copper – meaning a smelting plant could reclaim almost 160 metric tons of this semi-precious metal from its process water annually, for example.
The remaining weak acid undergoes another sulphide precipitation to remove arsenic and other heavy metals. The dewatered, and therefore weight-reduced arsenic sludge can then be disposed of at a hazardous waste disposal facility. Once these two precipitation stages are complete, the end result is an aqueous phase, primarily composed of sulphuric acid. In its untreated state, this can be used in the heap leaching process to reclaim copper from low-grade ore, for example. If required, additional nanofiltration can improve the quality of the sulphuric acid.
“Research has shown that by deploying this method, smelting plants can see a return on their investment in as little as four months,” explains Ante. The reasons for this are twofold: companies avoid unnecessary waste disposal costs, and generate revenues from reclaimed copper and sulphuric acid. Around 125 copper smelting facilities worldwide stand to benefit from this new technology. Applications at zinc and lead smelting plants are also conceivable; however, further research is needed to ensure that the desired metals can be selectively separated from the hazardous materials.
Events
IWA World Water Congress & Exhibition
Aug 11 2024 Toronto, Canada
Aug 25 2024 Stockholm, Sweden and online
Sep 03 2024 Mexico City, Mexico
Sep 03 2024 Mexico City, Mexico
Sep 03 2024 San Diego, CA, USA