-
 Geothermal energy is ideal to be combined with some DAC processes
Geothermal energy is ideal to be combined with some DAC processes
Green Energy
Carbon Capture and Hydrogen to Produce Green e-Fuels
Apr 29 2021
Author credit – Stephen B. Harrison, Managing Director, sbh4 consulting
Various mechanical direct air capture (DAC) processes have been developed to simulate the action of plants and capture carbon dioxide (CO2) directly from the air. In the past decade tremendous amount of research has been undertaken to scale up and commercialise these technologies.
Carbyon from the Netherlands, a Spin-Off from TNO, has a highly innovative and potentially efficient DAC technology. The proposed process is derived from photovoltaic research. It is based on a porous thin-film which is coated with amine- and/or bicarbonate-based adsorbents.
The combination of this thin-film, which is only a few microns thick, and a porous medium could prove to be very energy efficient. The pressure-drop for the air to cross the thin, porous medium is very small and the thermal mass of the thin film is low. These factors result in a very low heat demand for the process to capture CO2.
The company was founded in 2019 and a first demonstration unit is expected to run this year, in 2021. The features of this new approach are very promising, but still they need to be confirmed at scale.
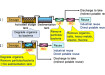
Another DAC innovator, Climeworks, was founded in 2009 as a Spin-Off from the ETH Zürich. In 2019 they acquired their Dutch competitor Antecy and integrated their technology. The Climeworks DAC equipment operates a repeating batch process.
In the first step of the Climeworks DAC process, air is blown through a collector by a fan. Some CO2 from the air is captured on the amine-based solid adsorbent in the collector and the CO2 concentration in the exhaust is significantly reduced. Once the adsorbent material is saturated, the collector is heated up to 80°C to 100°C. This releases the CO2 from the solid sorbent. The high purity carbon dioxide is collected for further processing.
Each Climeworks collector can capture up to 50 Tonnes of carbon dioxide per year, assuming a capacity factor of close to 100%. The actual performance is impacted by several parameters, including the ambient conditions and weather.
The specific energy demand is 2,000 kWh/Tonne CO2 of low-grade heat and 650 kWh/Tonne CO2 of electricity, which is mainly required to operate the fan that draws air across the sorbent material. Despite the heat demand being quite large, an advantage of the process is that it only needs heat at low temperature. If this can be recovered as waste-heat from an adjacent chemical or thermal process, the total primary heat demand can be significantly reduced.
Climeworks has sold and operates more than 14 DAC systems of various sizes worldwide. The largest project, with an annual capture capacity of 4,000 Tonnes of CO2, is under construction in Iceland. The captured CO2 will be permanently stored underground when the gas is mineralised to carbonates using the innovative Carbfix process. Heat and power for the system will be supplied from a geothermal power plant to ensure a negative carbon footprint to the overall scheme.
Beyond existing CO2 utilisation cases such as pH control in wastewater, beverage dispense and accelerated growing conditions in greenhouses, the production of e-fuels can be a major application of CO2 from DAC in the future. In the simplest case, captured carbon dioxide and hydrogen from an electrolyser are synthesised to methanol (CO2 + 3 H2 ïƒ CH3OH + H2O).
Methanol acts as a hydrogen carrier and remains liquid under ambient pressure and temperature. Thus, the storage and transport of methanol is much easier and cheaper than liquid or compressed hydrogen distribution. The methanol synthesis has an energy efficiency of about 80%. This is much better than generating cryogenic liquid hydrogen, which consumes about 30% of the lower heating value (LHV) of hydrogen. The energy efficiency of the methanol production is only slightly worse than high-pressure compressed hydrogen where the energy losses are between 10 and 15% of the LHV of hydrogen.
To produce methanol, the CO2 must be reacted with hydrogen which can be generated on an electrolyser from renewable power. The exhaust heat of the methanol synthesis at circa 200°C, and the exhaust heat from hydrogen production by electrolysis are at circa 80°C, can be used in the processes of low-temperature DAC. This smart heat integration would be beneficial for the overall methanol e-fuel production process efficiency.
In considering the merits of any renewable fuel value chain, neither efficiency nor specific heat demand alone will determine the viability of the process. The total costs associated with production, distribution and utilisation will be the governing factor.
Events
May 05 2024 Seville, Spain
May 13 2024 Munich, Germany
May 23 2024 Beijing, China
May 23 2024 Beijing, China
Jun 10 2024 Algiers, Algeria