-
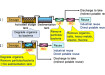
 Carbon powder is a co-product of methane pyrolysis and turquoise hydrogen production
Carbon powder is a co-product of methane pyrolysis and turquoise hydrogen production -
 Turquoise hydrogen is produced from methane pyrolysis
Turquoise hydrogen is produced from methane pyrolysis -
 The coat of arms of the City of Marl, Germany
The coat of arms of the City of Marl, Germany
Green Energy
20,000 Celcius plasma to produce low-carbon hydrogen
Jun 25 2021
Author credit – Stephen B. Harrison, sbh4 consulting
Low-carbon hydrogen is a clean energy vector that can help in the drive to reduce greenhouse gas emissions and combat climate change. The use of carbon capture to mitigate the CO2 emissions from hydrogen produced from fossil fuels is one route to produce low-carbon hydrogen. Green hydrogen produced on electrolysers fed with renewable electrical power is another.
Turquoise hydrogen produced by methane splitting, also known as methane pyrolysis or cracking results in no carbon dioxide emissions because the carbon atom from the methane molecule is released as solid carbon without CO2 gas emissions. It is therefore a potential pathway to low-carbon hydrogen production.
Methane pyrolysis requires energy to convert the methane to hydrogen and solid carbon. Biomethane can be used as a carbon-neutral feedstock and electrical heating, or plasma generated from renewable power can also minimise the CO2 footprint of turquoise hydrogen production.
In the city of Marl, Germany, an ultra-high temperature electrical plasma technology has been used for more than 80 years to crack hydrocarbons to produce chemicals such as acetylene. At present, about 20 Tonnes of hydrogen is produced per day as a co-product. The technology and know-how accumulated over these decades of operation can be applied to produce low-carbon turquoise hydrogen in the future.
Each plasma pyrolysis reactor is only 2m tall and 0.15m in diameter but is fitted with an extremely powerful 10MW arc torch. The plant is capable of processing 100 Tonnes per day of hydrocarbon feedstock, which is currently a C3 / C4 mixture. Lighter hydrocarbons, such as methane, would require more energy for their decomposition through pyrolysis.
Unlike some other plasma-based technologies for turquoise hydrogen which use graphite electrodes, the Marl process uses an electric arc that is generated between carbon steel electrodes. The maximum temperature of the plasma is 20,000 °C – more than three times hotter than the surface of the sun.
If the arc were to remain in one position, it would clearly melt the carbon steel components. So, movement of the arc is induced by the introduction of the feedstock in a way to create a vortex within the cylindrical reactor tube. The arc dances around to avoid over-heating the steel reactor chamber. This movement of the plasma arc ensures that the anode remains in the range of 400 to 450 °C and the cathode between 800 and 900 °C.
Gases leaving the reactor are quenched using a direct contact water spray. The temperature reduction terminates the reaction to optimise production of acetylene because, at Marl, that is the target gas. Acetylene is used to produce petrochemicals and the solid carbon powder is burned on a nearby power plant to release its energy value. If the carbon powder were to be processed into pellets, it could be used as a coke substitute in steel making.
The methane splitting reaction chemistry was described by Louis S. Kassel in his ‘Thermal decomposition of methane’ paper of 1932. The pathway is: methane to ethane (some hydrogen is released) to ethylene (more hydrogen is released) to acetylene (yet more hydrogen is released) to carbon (the final hydrogen atoms are split from the carbon atom). If hydrogen is the target, not acetylene, the reaction can be allowed to flow to completion.
At current natural gas prices in the region, the Marl process can be used to generate turquoise hydrogen at a cost in the order of €2/kg. That is substantially less than the cost of green hydrogen from electrolysis. At present, CCS is not possible in Germany. Neither the geology nor the regulatory environment facilitate that route for gaseous CO2emissions disposal. So, the option of producing blue hydrogen from natural gas reforming with carbon capture and storage is not realistic.
If the solid carbon can be used in a way that avoids CO2 emissions, the methane feedstock is from biogas and the power for the arc is from renewable electricity, the Marl process for turquoise hydrogen production could be a major contributor to a decarbonised energy system in central Europe.
Events
May 05 2024 Seville, Spain
May 13 2024 Munich, Germany
May 23 2024 Beijing, China
May 23 2024 Beijing, China
Jun 10 2024 Algiers, Algeria