Air Clean Up
Promising Step Towards Combating Global Warming
Aug 07 2012
Diamond Light Source (UK) has been chosen to improve low cost methods for carbon capture. Scientists from the University of Leeds are using the UK’s national synchrotron to investigate the efficiency of calcium oxide (CaO) based materials as carbon dioxide (CO2) sorbents. The results from this will help to provide a reason for one of the key mechanisms involved. It is hoped that this research will improve efforts in the efficiency and economy for carbon capture and storage.
Currently carbon capture occurs as the flue gases pass up the chimney, filtering out the CO2. This happens as the filter is a solvent which will absorb CO2, it is then heated and will release water to leave the CO2 behind. A better method is to filter CO2 out before combustion using a catalytic converter. This way the CO2 is not diluted into other flue gases. This can reduce power plants carbon emissions by around 80% to 90%.
CaO based materials have a large range of applications including pre- and post-combustion carbon capture technologies and thermochemical fuel upgrading. They are low cost, high abundance, have a large absorption capacity and fast reaction rates during the chemical process. The CO2 is captured when the temperature is around 400-800 °C by the formation of calcium carbonate (CaCO3). This can be regenerated causing the release of CO2 ready to be compressed and stored. However after multiple uses the capacity for capture reduces due to the loss of surface area. This surface area can be restored by hydration but this reduces the mechanical strength of the material. If these problems can be overcome, CaO based materials could provide a low cost answer for carbon capture on a very large scale.
Led by Dr Valerie Dupont and Dr Tim Comyn from the University of Leeds’ Faculty of Engineering, the team carried out a series of experiments on Diamond’s High resolution powder diffraction beamline, I11, using intense X-rays to study the carbon capture and hydration process in CaO based materials on the nano-scale. Their observations suggest a mechanism for the interaction between CaO and water during hydration.
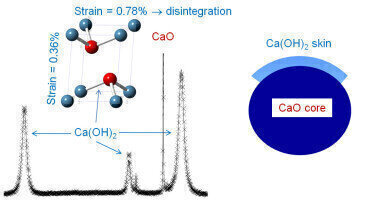
Dr Comyn explains, “We found that the stresses in the calcium hydroxide phase when bound to CaO were more than 20 times higher than its strength, leading to disintegration and the generation of nano-sized crystallites. Although the generation of a high surface area is a good thing, mechanical friability needs to be kept in check in order to achieve long term reliability for these systems.
“Our analysis provides an explanation of the enhanced capture/disintegration observed in CaO in the presence of steam. Now we understand this, the next step is to develop methods for improving the materials used, and apply the same techniques to other systems.”
Roger Molinder, an Engineering and Physical Sciences Research Council (EPSRC) funded PhD student on the project, describes, “Using the high resolution powder diffraction beamline at the Diamond synchrotron was key to this discovery; conventional X-ray sources such as those found at most Universities in the UK provide data with broad peaks, which do not make this sort of analysis possible. From a rigorous analysis of peak shapes arising from the data, we were able to determine the shape and size of the hydroxide phase, and determine the level of stress. Knowledge of these derived parameters is key to understanding the mechanism of sintering/disintegration.”
Concerns about global warming have prompted both national and international efforts to curb CO2 emissions. CaO based materials are a promising option for the removal of CO2 from flue gases at temperatures between 400 and 800 °C from processes such as fossil-fuel combustion. They can also be considered as a way of removing CO2 generated from thermochemical fuel upgrading with biomass sources - this is growing more popular as an alternative to fossil fuels. Using CaO based materials for carbon capture is just one of the ways the can be considered in an effort to combat global warming. Since CaO based materials are low cost, there is an economic incentive to solve the problem of surface area loss to potentially turn this into a method for large scale CO2 capture. These recently published results are a promising step towards improving these low cost methods.
Events
May 13 2024 Munich, Germany
May 23 2024 Beijing, China
May 23 2024 Beijing, China
Jun 10 2024 Algiers, Algeria
Jun 10 2024 Frankfurt, Germany